Pharmacology, Drug Delivery & Development
CFD Research has extensive experience in modeling and simulations of multiple GDUFA research priorities areas, especially those that involve complex routes of delivery, such as, nasal, inhalation, dermal, and ophthalmic drug routes. At present, we have three FDA funded research programs that strategically align with the proposed FDA-sponsored Center for Research on Generic Drugs.
Inhalation
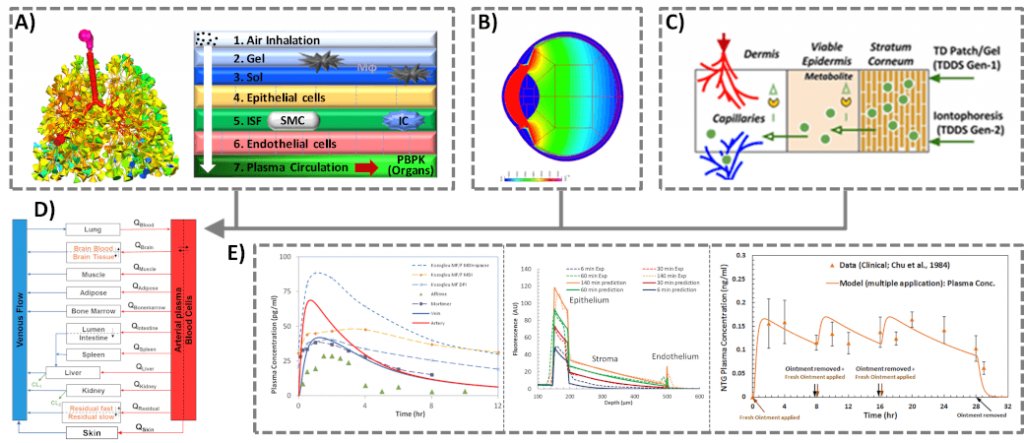
The goal is to develop and validate a computational framework to simulate pharmacokinetics of orally inhaled drug products (OIDPs). In this work, we have developed a novel quasi-3D modeling approach to simulate drug deposition in first of its kind full 24 generation in silico lung model. We have further linked these deposition models with whole-body PBPK models. Such integrated models will help determine the bioequivalence for OIDPs without the need for comparative clinical endpoint studies. [1-3]
Ophthalmic
The goal is to develop a unique multiscale- multiphysics model to simulate ocular delivery, absorption, distribution, pharmacokinetics and in vitro to in vivo extrapolation (IVIVE) for generic drugs and drug products. Our developed approach account for the complex anatomy and physiology of the eye, physicochemical material properties of ocular tissues and barriers, and physicochemical drug/carrier properties. These models can provide an accurate and efficient analysis to virtually test, design, and develop ocular drug products and to facilitate translational applications from bench to bedside, eliminating the high cost and time of in vitro and in vivo experiments. [4]
Dermal
The goal is to develop a multiscale simulation toolkit for computational pharmacology of trans-intradermally administered compounds (CPDAC) in healthy and diseased skin population. The developed toolkit involve the quantitative structure activity relationship QSAR-based generalized constructs to automate the action of chemical penetration enhancers on drug permeation, models of field assisted iontophoresis, and 3D models of healthy and diseased skin barriers and microvasculature. The intended CPDAC outcome can be used to perform virtual bioequivalence studies of topical products that will allow the evaluation of the impact of formulation differences on the in vivo performance of dermatological drug products and cosmetics. [5]
References
- Kannan et al (2018) A compartment-quasi-3D multiscale approach for drug absorption, transport, and retention in the human lungs. Int J Numer Method Biomed Eng. (PMID: 29272565)
- Kannan et al (2017) A quasi-3D wire approach to model pulmonary airflow in human airways.. Int J Numer Method Biomed Eng. (PMID: 27704716)
- Kannan et al (2018) A Quasi-3D compartmental multi-scale approach to detect and quantify diseased regional lung constriction using spirometry data. Int J Numer Method Biomed Eng. (PMID: 29486525)
- Pak J, et al (2018) Computational modeling of drug transport across the in vitro cornea. Comput Biol Med. (PMID: 29175100)
- Somayaji, M.R; Das, D; Chen, Z; Harrand, V.J; Przekwas, A.J; Simon, L.S; Prausnitz, M; Murthy, S.M. , A Simulation Toolkit for Pharmacotherapy of Dermally Administered Compounds, in Annual CRS Meeting. 2018: New York.